EudraLex Volume 10 Updates: What They Mean for Future EU Clinical Trials
The European Commission has updated EudraLex Volume 10, reshaping how clinical trials are conducted across the EU. These changes reflect the full implementation of the Clinical Trials Regulation (CTR) EU No 536/2014, and they carry major implications for CROs, sponsors, and biotech/pharma companies planning new studies.
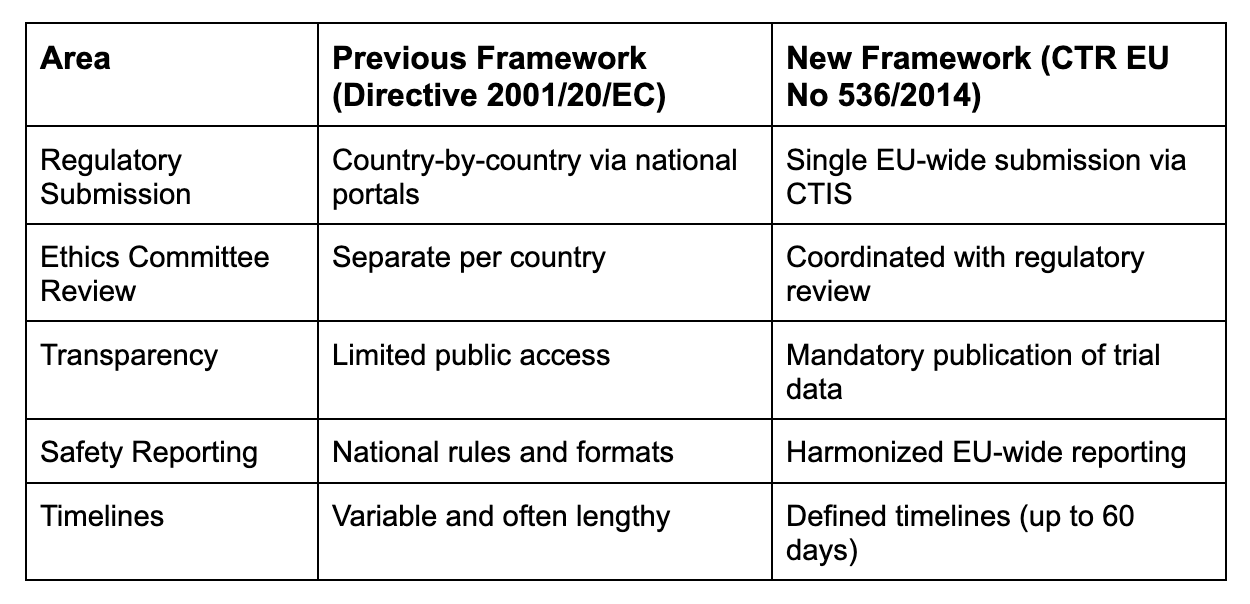
Key Changes at a Glance
What This Means for Sponsors and CROs
1. Centralized Submissions via CTIS
The Clinical Trials Information System (CTIS) is now the mandatory portal for all EU trial applications. Sponsors must prepare harmonized documentation and navigate a single submission process, streamlining approvals but requiring greater precision.
Implication: CROs must be CTIS-proficient and able to manage multi-country submissions with synchronized timelines.
2. Integrated Ethics and Regulatory Review
Ethics committees now work in tandem with regulatory authorities, reducing duplication and speeding up decisions.
Implication: Sponsors must align ethical and scientific documentation early, and CROs must coordinate stakeholder engagement across jurisdictions.
3. Enhanced Transparency
Trial protocols, summaries, and results are now publicly accessible via CTIS, even for early-phase studies.
Implication: Sponsors must balance transparency with IP protection, and CROs must ensure accurate, timely disclosures.
4. Safety Reporting Standardization
Serious adverse events and annual safety reports follow unified EU formats, improving pharmacovigilance oversight.
Implication: CROs must upgrade safety systems and ensure compliance with new reporting templates and timelines.
5. Defined Timelines and Milestones
The CTR enforces strict review deadlines, typically 45 days for initial assessment, extendable to 60 days with queries.
Implication: Sponsors must plan trial readiness meticulously, and CROs must manage documentation and responses with precision.
Strategic Considerations for New Trials
Protocol Harmonization: Design trials that meet EU-wide standards while accommodating national nuances.
Site Selection: Choose sites with CTIS experience and ethics committee alignment.
Digital Readiness: Invest in systems that support CTIS workflows, safety reporting, and public disclosures.
Partner Expertise: Work with CROs and consultants who understand the CTR’s operational and strategic impact.
Altiveno Life Sciences offers tailored support for navigating these changes, from CTIS strategy to ethics coordination and safety compliance.
Ready to future-proof your EU clinical trials? Let’s talk.